Clean hydrogen offers the only long-term, scalable, and cost-effective option for deep decarbonization in sectors such as steel, maritime, aviation and ammonia, contributing 20% of the total abatement needed in 2050.
Hydrogen Basics
What’s Special about green hydrogen?
Long term
Zero CO2
Today, about 95% of total Hydrogen supply comes from fossil fuels, through a process called steam methane reforming (SMR), generating several amounts of CO2 per year
Competitive
With latest technological improvements, availability of renewable energies and market prices in gas, CO2 and energy, green hydrogen is starting to be competitive today versus conventional alternatives for some end applications such as refineries, ammonia, steel and long-haul and heavy duty vehicles.
Green hydrogen?
An everlasting clean energy source which is paramount for a long lasting energy transition and fundamental to achieve a real change
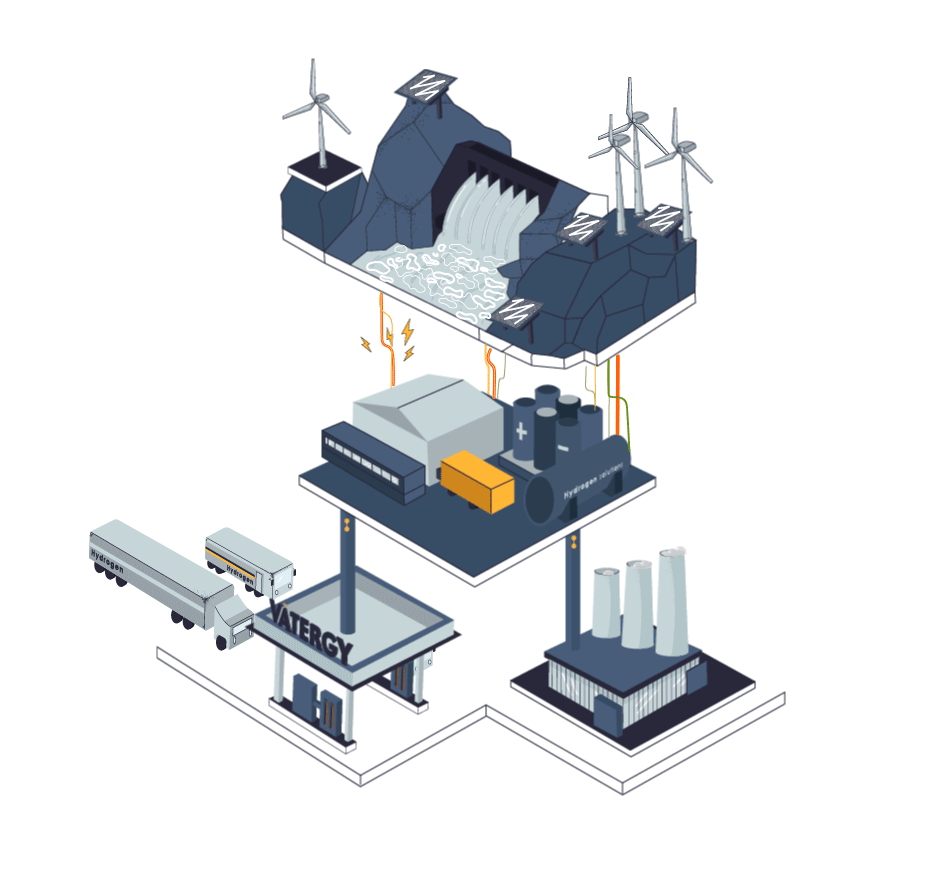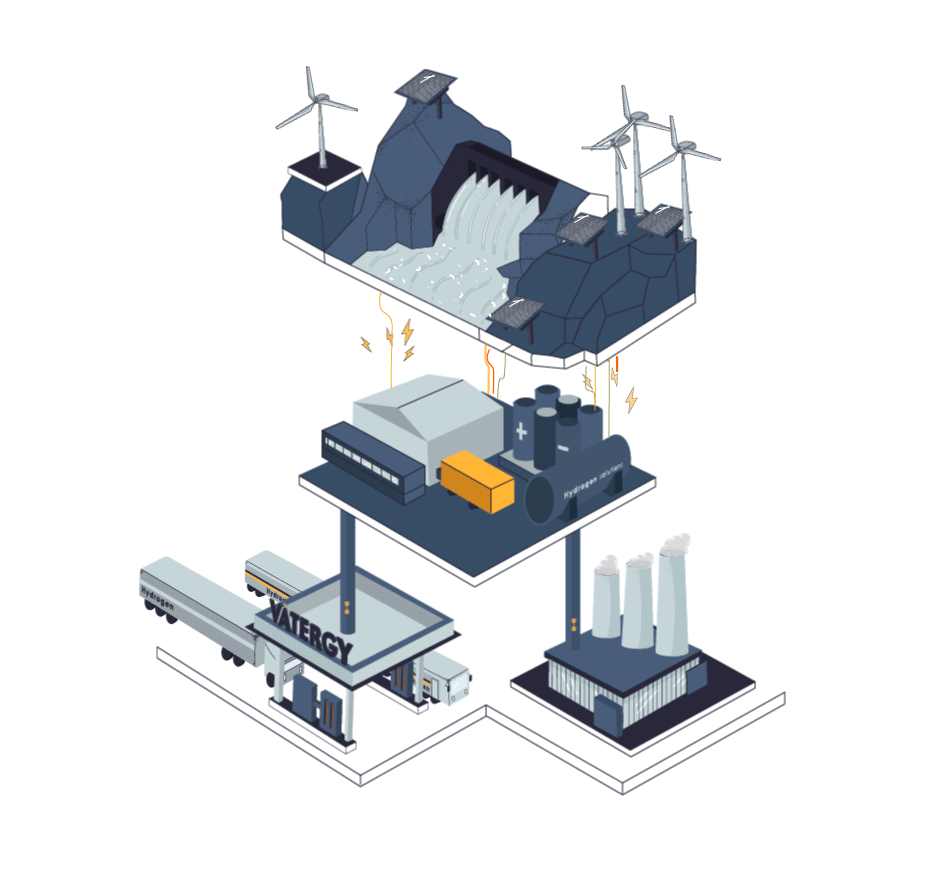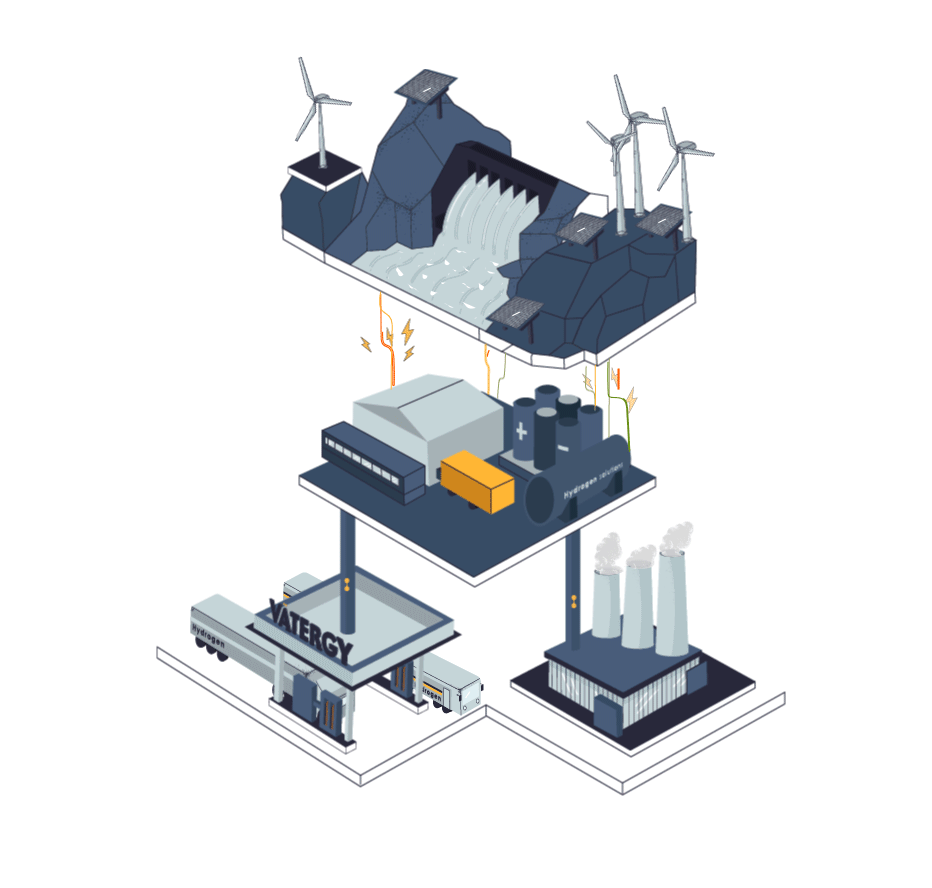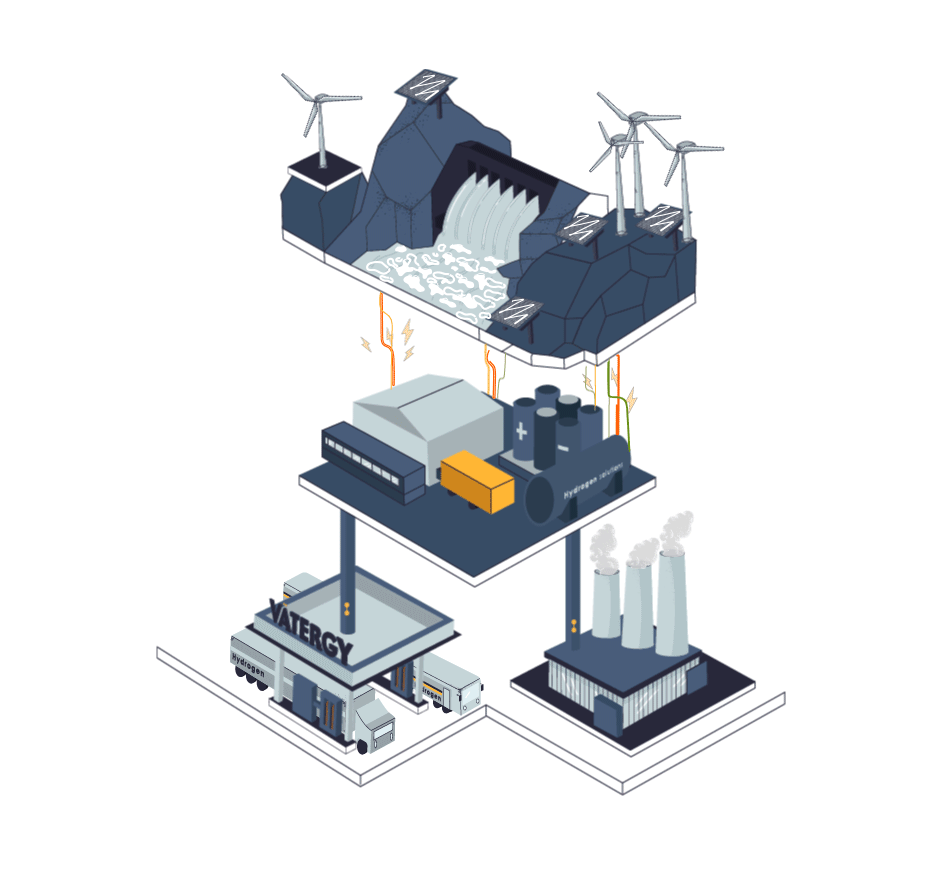
Some industries cannot be electrified. They need flame, a heat curve or a chemical reaction that is not possible in a pure electric process. Those industries today use fossil fuels for their processes, or grey hydrogen produced from methane.
Clean hydrogen offers the only long-term, scalable, and cost-effective option for deep decarbonization in sectors such as steel, maritime, aviation and ammonia, contributing 20% of the total abatement needed in 2050.
It is impossible to achieve the net-zero emissions targets set for 2050 without clean hydrogen. Pure electrification is not enough.
Hydrogen basics
Clean Energy

The production of hydrogen through electrolysis requires a lot of electricity: About 52 Kwh to produce 1kg of H2, which is the equivalent of having 23 domestic ovens turned on for an hour.
Therefore, in order to ensure that the whole process is completely free of greenhouse gas emissions, the energy used should be clean.
Clean energy is energy that is produced from renewable, zero emission sources that do not contaminate the atmosphere when used.
The most common types of clean energy are hydropower, geothermal, wind and solar.
Although clean energy is fantastic, it has some limitations: the sun does not always shine, and wind does not always blow. Furthermore, it is not easy to store energy surplus when production surpasses the demand at a given time. Green Hydrogen can help solve this issue serving as storage: Power can be converted to hydrogen when there is a surplus on the supply and hydrogen can be converted back to power when it is needed. All of this with ZERO EMISSIONS.
Hydrogen basics
Water
Water is the feedstock needed to generate green hydrogen. Each molecule of water contains one oxygen and two hydrogen atoms.
Water is also the main constituent of the Earth’s hydrosphere covering approximately 70% of the Earth’s surface.
Through Electrolysis, and based on the atomic properties of water, 1 kg of hydrogen gas requires about 2.4 gallons of water as feedstock.
The good news is that electrolysis can use purified industrial or sewage water in the process, minimizing the need of potable water as feedstock
Hydrogen basics
Electrolysis
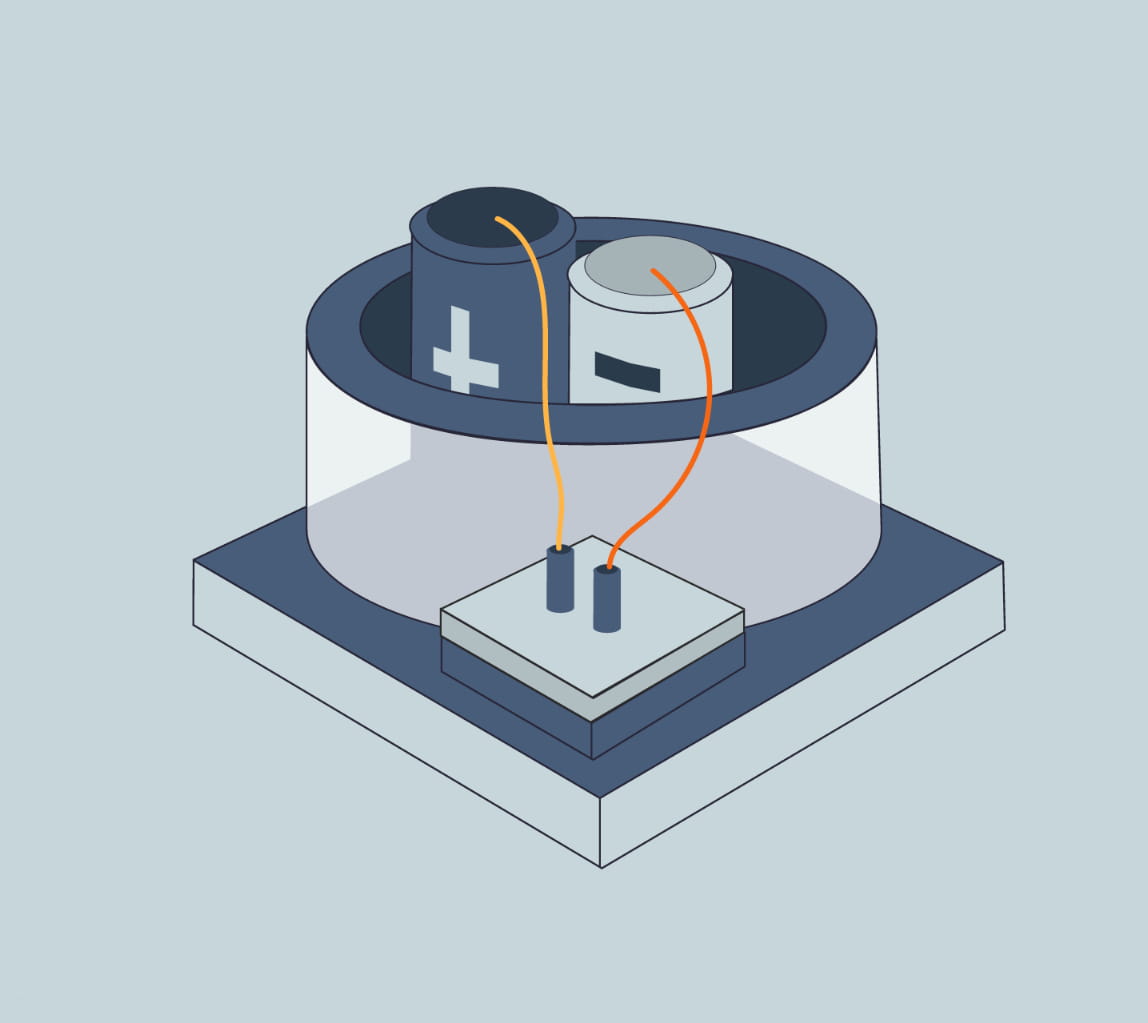
The process to produce Hydrogen from electricity and water is called electrolysis. In simple terms, electrolysis is a technique that uses electric energy to drive a chemical reaction that otherwise would not spontaneously happen. In this specific case, renewable energy is used to drive the separation of the water molecules (H2O) into Hydrogen and Oxygen gas.
The device to perform this process is called Electrolyser.There are three main types of electrolysers in water electrolysis hydrogen production process technology: alkaline electrolytic cells (alkaline), Proton Exchange Membrane (PEM), and solid oxide electrolytic cells (SOEC).
Hydrogen basics
Downstream

Through electrolysis, energy is converted from electrons to hydrogen molecules.
Hydrogen, similarly to natural gas, can be stored and transported in liquid or gas forms. To exist as a liquid, hydrogen needs to be cooled below −253 °C which is significantly lower than the temperature required to liquify natural gas (LNG): at approximately −162 °C, therefore requiring an enormous amount of energy to liquify it.
Today, logistics of hydrogen represents a big challenge as the volumetric energy density is quite low compared to other traditional fuels. Nevertheless, today, hydrogen can be safely stored, distributed (trucks or pipes) and used in different industrial and mobility applications.